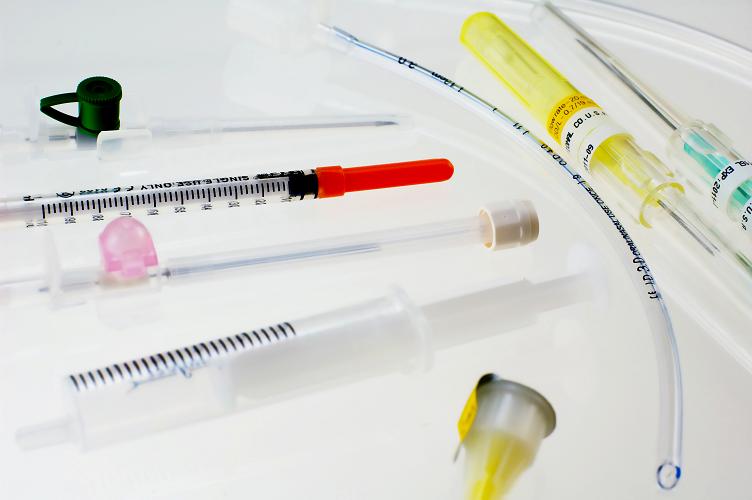
Medical Device Pad Printing
TouchMark is the medical device marking company that customers have come to trust for high resolution permanent markings on difficult substrates using USP Class VI medical grade and Biocompatible Inks.
TouchMark offers a variety of print capabilities including pad printing, screen printing, and digital printing, along with a variety of custom specialty inks such as: Biocompatible Inks, Radiopaque Inks and Conductive Inks.
Medical Device Pad Printing/Marking Examples:
- Microcatheters
- Catheters
- Various Tubing
- Hypo Tubes
- Various Tubes
- Handles
- Trocars
- Clips
- Adapters
- Shafts
- Syringes
- Housing
- Cannula
- Guide Wires
- Connectors
- Lenses
And other devices in various medical fields:
- Vascular Devices
- Orthopedic Devices
- Dental Devices
- Neurological Devices
- Ophthalmologic Devices
- Pharmaceutical
- ENT Devices
Prototyping and Production Volume
Servicing the medical diagnostic industry for over 20 years, TouchMark’s engineering staff works closely with the customer’s Research and Development department to assure that the proper materials (PEEK, Polyimide, Nylon, Acrylic, Polycarbonate, Polystyrene, ABS, etc.) and best medical grade inks are properly matched for the device application. We work with you from the design phase to high volume production runs.
Testing protocols and specifications are developed as part of the first article prototypes which enables the printing process to ramp up to full scale manufacturing while preserving the customer’s cost and lead time requirements.
Lens Pad Printing Available
